Abstract
BACKGROUND: Sickle RBCs generate excessive reactive oxygen species (ROS) due to increased HbS auto-oxidation and cell lysis. High plasma ROS lead to increased oxidative stress in the brain, where oxidative stress is linked with the etiology of a host of cerebral disorders. Noninvasive assessment of cerebral oxidative stress could be useful in understanding how ROS affects the brain in sickle cell disease. We examined a blood-brain barrier-permeable and stable nitroxide, methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (MCP), which is a paramagnetic T1-shortening MRI contrast agent in its oxidized state. MCP participates in cellular redox reactions (is reduced by free radicals, ubiquinols, ascorbate, glutathione (GSH), etc.) and once reduced, it loses its ability to generate MRI contrast (becomes diamagnetic). MCP is highly lipophilic, and enters the brain rapidly after intravenous infusion. The rate of loss of contrast within the brain tissue after intravenous infusion is related to tissue ROS load. We employed MCP to evaluate the cerebral ROS levels in transgenic-knockout sickle mice.
METHODS: BERK transgenic mice (n=3) and C57BL/6 (n=3, WT) were studied by MRI at 9.4 Tesla under isoflurane anesthesia. MCP was administered intravenously as a bolus (25 ul/s of an 85 mmol/l solution in dionized water) at a dose of 0.5 mmol/kg BW (one WT was IVI at 0.4 mmol/kg BW). Dynamic T1 inversion recovery (T1IR) imaging (20 seconds/T1 assessment) was performed prior to ( for 6 minutes) and after (for 27 minutes) MCP infusion. T1 relaxivity kinetics were fit using 2-compartment model. Cerebral blood flow (CBF) and cerebral metabolite concentration were estimated in a different group of BERK (n=10) and control mice (n=11) (Table 2) via in vivo MRI/MRS approach (same anesthesia). In vitro assessment of the MCP T1-dependence on concentration permitted in vivo determination of MCP concentration (data not shown).
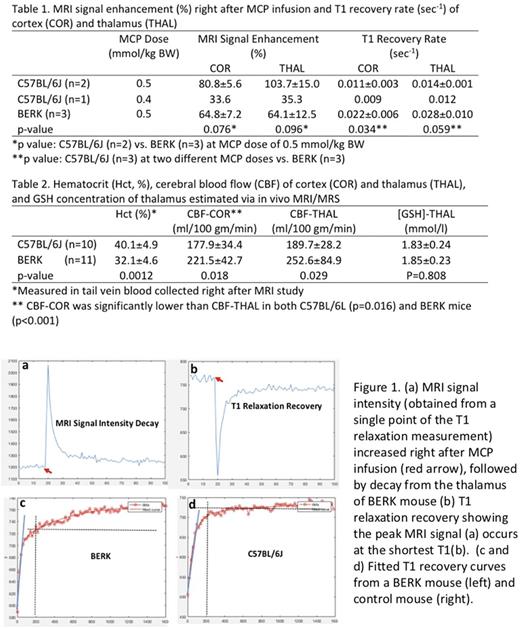
RESULTS: MRI signal intensity peaked immediately after the injection of MCP (Figure 1a), due to MCP's T1 shortening effect (Figure 1b). In control mouse at a MCP dose of 0.5 mmol/kg BW, signal increased ~80% and ~100% immediately after MCP infusion in the cortex (COR) and thalamus (THAL) regions, respectively. In BERK mice, the signal peaked at lower levels than in control mice.
The rate at which T1 signal recovered in BERK mice was significantly more rapid than in control mice ( as shown by the steeper slope of the T1 recovery curve, indicated by the blue line in Figure 1 c, d). Signal recovery was prolonged in the BERK mice, with a slow second phase of recovery evident 200 seconds after MCP infusion (note progression of recovery after the gray dotted verticle line). The fast phase of T1 recovery in BERK cortex was double that of control mice (Table 1), and a similar trend was also observed in thalamus. A faster rate of recovery is attributed to increased MCP reduction. Peak intensity, but not rate of recovery, was affected by lower dose in the one animal (Table 1).
Conslusion and Discussion: In the blood, MCP reduction begins immediately after IVI, suggesting that some MCP never reaches the brain in the oxidized (paramagnetic) state. This determines the "peak" contrast change observed, which was lower in the BERK mice with higher blood ROS. In the brain, MCP is reduced by various reductants, forming hydroxylamine and/or oxoammonium (Zhelev Z 2013 ACS Chem Neurosci), loosing its paramagnetism, so T1 returns to pre-infusion levels. Notably, the T1 recovery rate in BERK mice suggested rapid reduction of the MCP in comparison to WT mice, implying a significantly higher oxidative stress load in BERK.
The MCP reduction rate is the sum of two opposing processes, i.e., the reduction of MCP to hydroxylamine and the reoxidation of hydroxylamine by ·OH to MCP, especially under iron overload (Okajo A 2009 Biol Pharm Bull). This reoxidation of hydroxylamine to MCP may explain the slow second phase predominantly observed in BERK mouse (Figure 2c). This also indicates higher free radical levels in BERK mouse brain. GSH does not appear to play a major role in MCP contrast since GSH concentration in BERK mouse brain is not different from that in control mouse as estimated using in vivo MRS (Table 2).
We have demonstrated for the first time increased oxidative stress in the sickle BERK mouse brain using in vivo redox imaging. These methods can provide a means to monitor the effect of antioxidants or therapy upon brain pathology in SCD mice.
.
Acharya: AIMA: Consultancy. Billett: Janssen Pharmaceutical: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal